 |
| This post was peer reviewed. Click to learn more. |
 |
| Image Credit: Flickr |
Author: Alexandria Gregory, MS3
Overview
An 80-year-old female with a history of chronic obstructive pulmonary disease (COPD), high cholesterol, and hypertension presented to the emergency department (ED) with a two-day history of shortness of breath. She also reported mild left-sided chest pain, but had no cough, fever, or calf pain. She had no history of deep vein thrombosis (DVT) or pulmonary embolism (PE), though she recently traveled from Massachusetts to Florida via airplane, and returned on the day her symptoms began. The patient had quit smoking over ten years prior to her presentation in the ED. She had been using her inhalers, prescribed for COPD, frequently with minimal improvement.
On exam, the patient was in mild respiratory distress with accessory muscle use. Breath sounds were diminished throughout with crackles at the bases. She became increasingly dyspneic as well as hypoxic while ambulating.
Diagnosis and Management
An electrocardiogram (EKG) was done in triage and showed inverted T waves in leads II, III, aVF, and V3-V6, which were new compared to a prior EKG. Workup was therefore focused on ruling acute coronary syndrome (ACS) as well as PE given the patient’s history. Labs, including troponin and brain natriuretic peptide (BNP), were ordered, as well as chest x-ray. Ventilation-perfusion (VQ) scan was also ordered, as patient could not undergo computed tomography angiogram due to impaired kidney function.
Troponin was elevated at 4.066 ng/mL and BNP was 2,198 pg/mL. The case was discussed with cardiology, and the decision was made to anticoagulate with heparin, as this would cover both ACS and PE. VQ scan ultimately showed low probability of PE. Echocardiogram was also added, which showed ejection fraction (EF) of 25-30% and was suggestive of Takotsubo (stress) cardiomyopathy. Cardiology recommended 40 mg intravenous furosemide and planned to do cardiac catheterization the following day. The patient was subsequently admitted to telemetry.
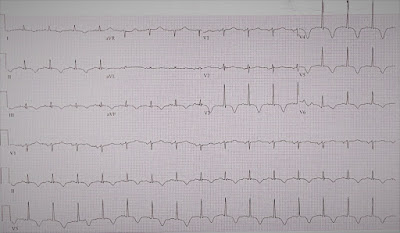 |
| Figure 1: Electrocardiogram noting T wave inversions in the inferior and anterolateral leads. |
Discussion
Takotsubo cardiomyopathy, also referred to as stress cardiomyopathy and broken heart syndrome, is characterized by transient systolic dysfunction of the left ventricle. While it mimics the presentation of myocardial infarction, it occurs in the absence of plaque rupture or obstruction of the coronary arteries. Frequently, the area of dysfunction extends beyond the area perfused by a single coronary artery.[1] Takotsubo cardiomyopathy is more common in women, and occurs in 1-2 percent of patients in which ACS or ST-elevation myocardial infarction is suspected and troponin is positive.[2]
The pathogenesis of stress cardiomyopathy is not well understood; however, there are a few main mechanisms that have been theorized to play a role. First, it has been proposed that catecholamine excess may result in myocardial stunning or direct myocardial toxicity.[3,4] Second, it has been suggested that mid-cavity or left ventricular outflow tract obstruction may cause apical dysfunction. Other proposed mechanisms include coronary artery spasm and microvascular dysfunction.[5] Often, Takotsubo cardiomyopathy is triggered by emotional or physical stress.
Stress cardiomyopathy most often presents with retrosternal chest pain, but may also present with dyspnea or syncope. Patients may also develop symptoms of heart failure, arrhythmias, cardiac arrest, or mitral regurgitation.[6] EKG often shows ST segment elevation, but may also show ST depression, QT prolongation, T wave inversion, or other nonspecific changes. Other useful tests that are often elevated are troponin and BNP.[6] Ultimately, definitive diagnosis requires all four of the following:
- Transient left ventricular systolic dysfunction
- Absence of obstructive coronary disease or angiographic evidence of acute plaque rupture
- New EKG abnormalities (ST elevation and/or T wave inversions) OR modest elevation in troponin
- Absence of pheochromocytoma or myocarditis
Conclusion
The following day, the patient underwent cardiac catheterization, which showed clear coronary arteries, supporting the diagnosis of Takotsubo cardiomyopathy. Further history revealed that the patient’s flight had been particularly stressful, as she had a nine-hour delay and is typically anxious in airports. This was felt to be the inciting event for her cardiomyopathy. During admission, the patient was started on a beta-blocker and given furosemide for diuresis given her elevated blood pressure and depressed EF. She was ultimately discharged home to continue the beta-blocker and furosemide and to follow up with cardiology for repeat echocardiogram to ensure improving EF.
References:
1. Sato H, Taiteishi H, Uchida T. Takotsubo-type cardiomyopathy due to multivessel spasm. In: Clinical aspect of myocardial injury: From ischemia to heart failure, Kodama K, Haze K, Hon M (Eds), Kagakuhyouronsha, Tokyo 1990.
2. Prasad A, Dangas G, Srinivasan M, et al. Incidence and angiographic characteristics of patients with apical ballooning syndrome (takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial: an analysis from a multicenter, international study of ST-elevation myocardial infarction. Catheter Cardiovasc Interv 2014; 83: 343-8.
3. Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006; 27.1523-9.
4. Nef HM, Möllmann H, Kostin S, et al. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J 2007;28.
5. Wittstein IS, Thiemann DR, Lima JA, et al. Neurohumeral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005; 352.
6. Hachamovitch R, Chang JD, Kuntz RE, et al. Recurrent reversible cardiogenic shock triggered by emotional distress with no obstructive coronary artery disease. Am Heart J 1995; 129.
